
This arrangement is obtained on the basis of following rules, They have diffused shapes.Įlectronic Configuration of Atoms: The arrangement of electrons in various orbitals is called the electronic configuration.
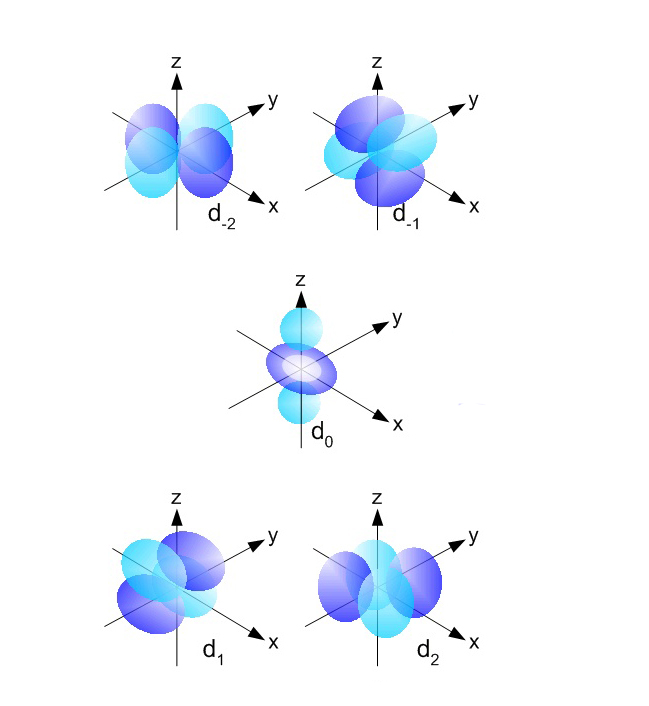
i.e., there are seven possible orientations for f orbitals. Boundary surface diagrams for d-orbitals are as follows:įor f-orbitals, Ɩ = 3 and m Ɩ = -3, -2, -1, 0, +1, +2 and +3. For d-orbitals, the number of radial nodes is 2 and the total number of nodes is n-2. The five d- orbitals have equivalent energies. The shapes of the first four d-orbitals are double dumb-bell and that of the fifth one, d z 2, is dumb-bell having a circular collar in the xy-plane. So there are 5 types of d-orbitals. They are d xy, d yz, d zx, d x 2 -y 2, and d z 2. i.e., there are five possible orientations for d orbitals. The diagrams for three 2p orbitals are as follows:įor d-orbitals, Ɩ = 2 and m Ɩ = -2, -1, 0, +1 and +2. All the p-orbitals have a dumb-bell shape. For p x orbital, the lobes are along the x-axis, for p y, they are along the y-axis, and for p z, they are along the z-axis. They differ only in the orientation of the lobes. The size, shape, and energy of the three orbitals are identical.
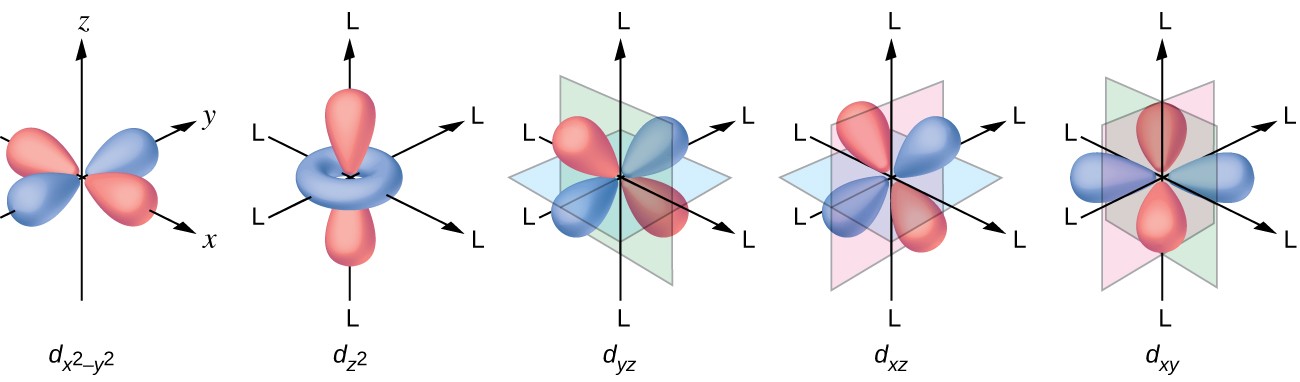
The probability density function is zero on the plane where the two lobes touch each other. So, there are 3 types of p-orbitals – p x, p y, and p z. i.e., there are three possible orientations for p orbitals. Shapes of Orbitals and Electronic Configuration All the s-orbitals are spherically symmetrical and their size increases with an increase in n.


 0 kommentar(er)
0 kommentar(er)
